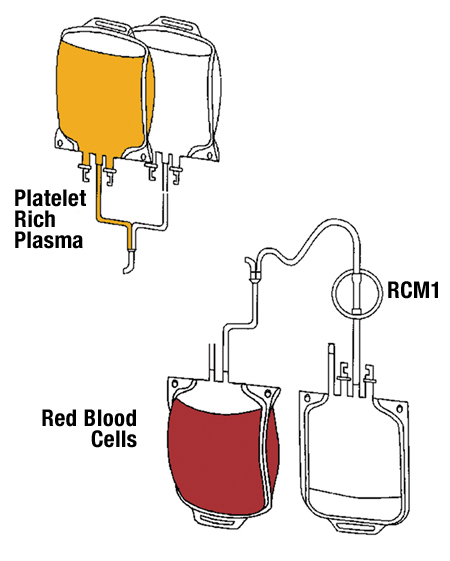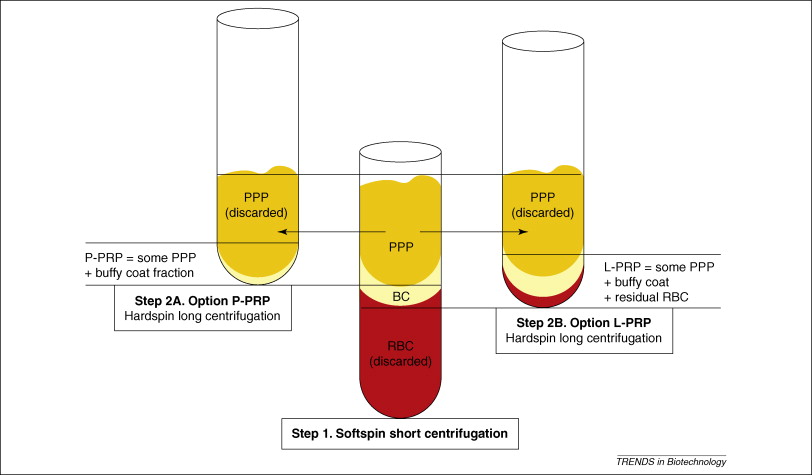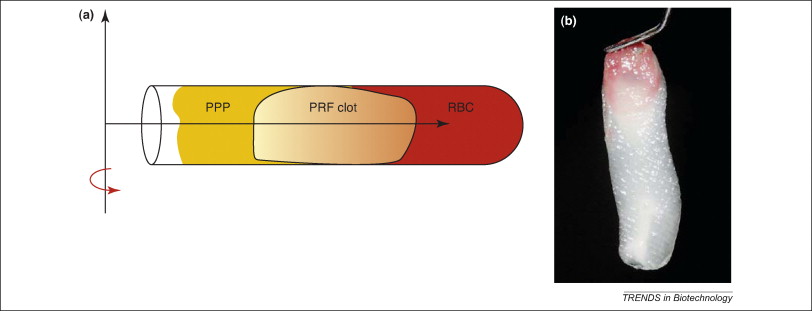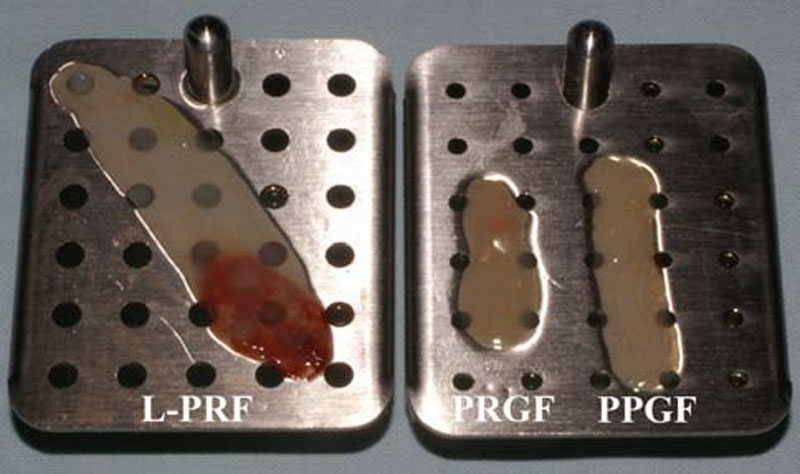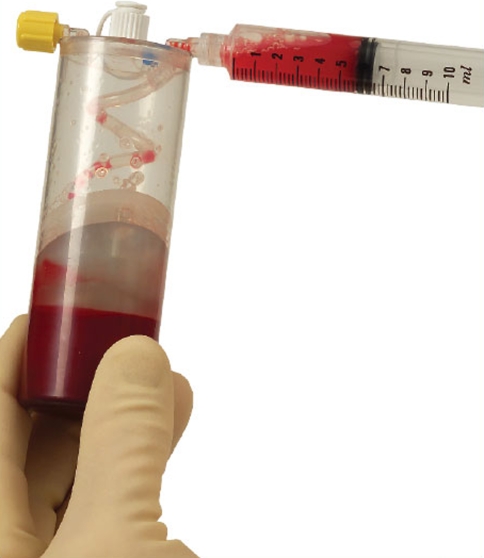
Platelet rich plasma Injection grafts for musculoskeletal injuries:a review(6).
Summary
In summary, for over 20 years PRP has been used safely in a variety of conditions with promising implications. Unfortunately, most studies to date are anecdotal or involve small sample sizes. Undoubtedly we are seeing increased clinical use of PRP, however more clinical trials are certainly needed. Little is documented in the literature regarding the expected timeframe of tendon healing post-PRP injection. Also, there are no studies to date that review the need of post-PRP injection rehabilitation, nor are there any protocols. However, it is assumed that Physical/Occupational therapy and restoring the kinetic chain will help facilitate recovery post injection.
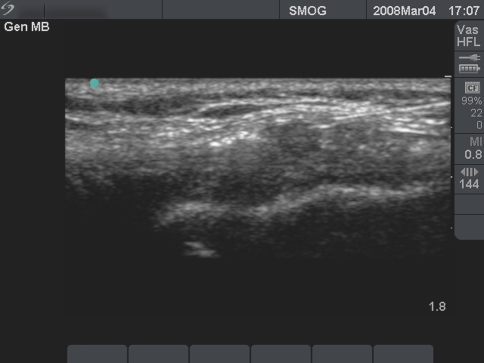
The authors are currently expanding PRP injection applications from tendon injuries to other persistent ailments including greater trochanteric bursitis and knee osteoarthritis with favorable results. The authors also have had success in injecting professional soccer athletes with acute MCL knee injuries in an effort to accelerate their return to play (Fig. 8). Further understanding of this promising treatment is required to determine which particular diagnoses are amenable to PRP therapy. The authors will report results on this topic in the near future.
Fig. 8
Ultrasound guided knee MCL injection/graft
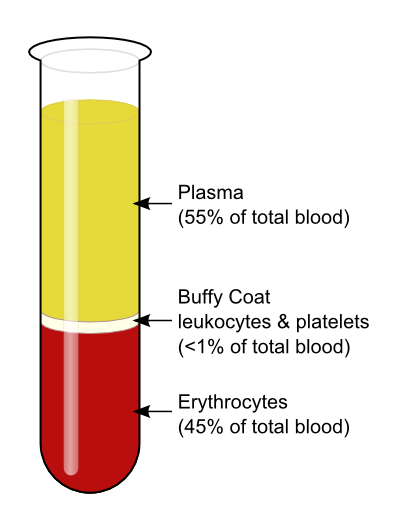
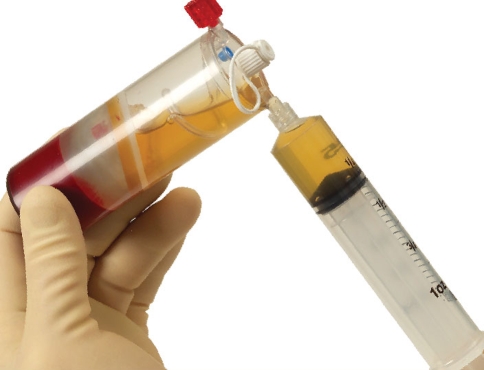
The use of autologous Growth factors in the form of PRP may be just the beginning of a new medical frontier known as “Orthobiologics.” First generation injectables such as Visco-supplementation have been successful in the treatment of pain for patients with osteoarthritis of the knee. These injections represent a non-biologic effort to influence the biochemical environment of the joint.
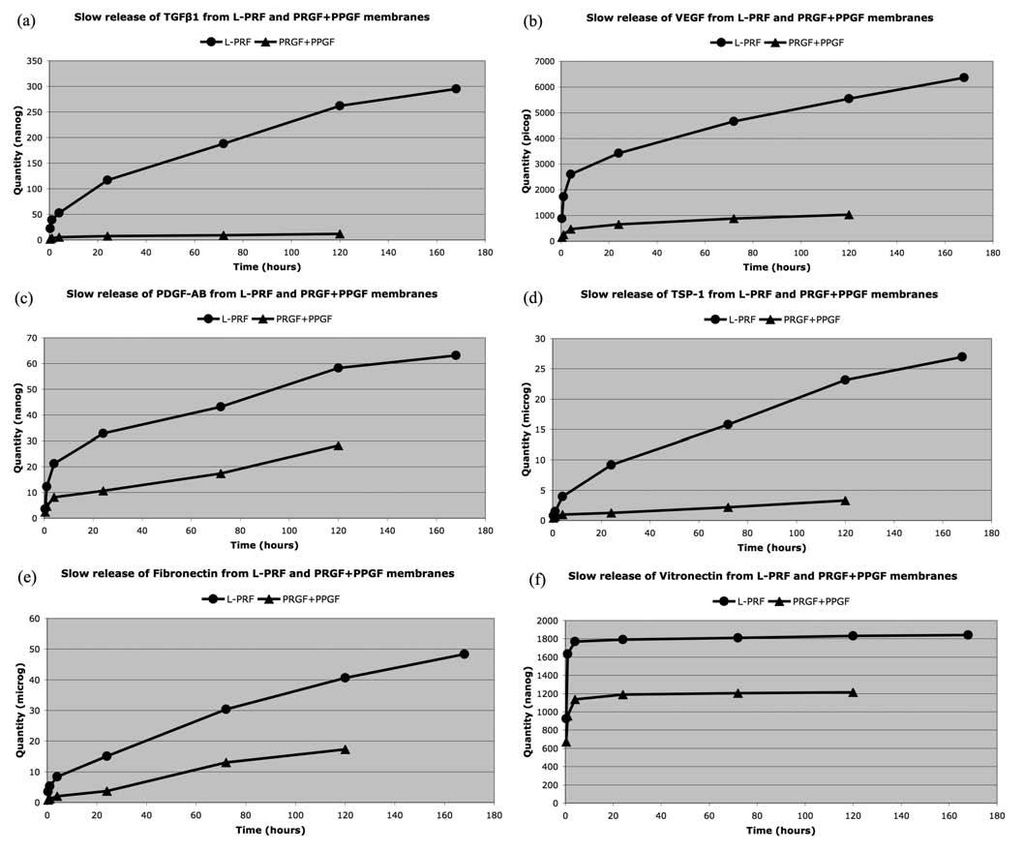
A second generation of injectables is now available with PRP. This technology provides delivery of a highly concentrated potent cocktail of Growth factors to stimulate healing. TGF-b, contained in PRP has been linked to Chondrogenesis in cartilage repair [43]. New reports presented at the 2007 International Cartilage Repair Society Meeting in Warsaw indicate PRP enhancement of Chondrocyte cell proliferation and positive clinical effects on degenerative knee cartilage [44, 45]. Anitua and Sanchez recently demonstrated increased Hyluronic acid concentration balancing angiogenesis in ten osteoarthritic knee patients [46]. Wu et al. documented PRP promotion of Chondrogenesis as an injectable scaffold while seeded with chondrocytes in rabbit ears. Hard knobbles were found and seen on MRI, as well as histologic analysis and staining which confirmed cartilage growth [47].
Future generations of biologic injectables may target specific cells, rather than providing an assortment of non-specific healing properties. Currently clinical trials of intra-articular use of growth factor BMP 7 (OPI) are underway. Soft tissue applications of BMP7 (OPI) are also in its early stages. Bone marrow aspirate stem cell injections are seeing increased clinical use as well. Ultimately, Stem cell therapy represents the greatest biologic healing potential.
References
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;10(10):1–5. [PubMed]
2. Barrett S, Erredge S. Growth factors for chronic plantar fascitis. Podiatry Today. 2004;17:37–42.
3. Woolf AD, Pfleyer B. Burdon of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed]
4. Anitua M, Sánchez E, Nurden A, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24(5):227–34. doi: 10.1016/j.tibtech.2006.02.010. [PubMed] [Cross Ref]
5. Praemer AF. Musculoskeletal conditions in the United States. 2. Rosemont: American Academy of Orthopaedic Surgeons; 1999.
6. Marx R, Garg A. Dental and craniofacial applications of platelet-rich plasma. Carol Stream: Quintessence Publishing Co, Inc.; 2005.
7. Everts P, Knape J, Weirich G, Schonberger J, Hoffman J, Overdevest E, et al. Platelet-rich plasma and platelet gel: a review. JECT. 2006;38:174–87. [PubMed]
8. Pietrzak W, Eppley B. Scientific foundations platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–54. doi: 10.1097/01.scs.0000186454.07097.bf. [PubMed] [Cross Ref]
9. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–8. [PubMed]
10. Ferrari M, Zia S, Valbonesi M. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47–50. [PubMed]
11. Antitua E, Andia I, Sanchez M, Azofra J, Del Mar Zalduendo M, Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF productions by human tendon cells in culture. J Orthop Res. 2005;23:281–6. doi: 10.1016/j.orthres.2004.08.015. [PubMed] [Cross Ref]
12. Fenwick SA, Hazlelman BL, Riley GP. The vasulature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252–60. doi: 10.1186/ar416. [PMC free article] [PubMed] [Cross Ref]
13. Hayem G. Tenology: a new frontier. Joint, Bone, Spine. Rev Rhum. 2001;68:19–25. [PubMed]
14. Jobe F, Ciccotti M. Lateral and medial epicondylitis of the elbow. J Am Acad Orthop Surg. 1994;2:1–8. [PubMed]
15. Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. Am J Hand Surg. 2003;28(2):272–8. doi: 10.1053/jhsu.2003.50041. [PubMed] [Cross Ref]
16. Antiua E, Sanchez M, Nurden A, Zalduendo M, Fuente M, Prive G, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res Pt A. 2006;77(2):285–93. doi: 10.1002/jbm.a.30585. [PubMed] [Cross Ref]
17. Kader D, Sakena A, Movin T, Magulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–49. doi: 10.1136/bjsm.36.4.239. [PMC free article] [PubMed] [Cross Ref]
18. Smidt N, Assendelft W, Arola H, et al. Effectiveness of physiotherapy for lateral epicondylitis: a systemic review. Ann Med. 2003;35:51–62. doi: 10.1080/07853890310004138. [PubMed] [Cross Ref]
19. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. [PubMed]
20. Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43:65–92. doi: 10.1016/S0169-409X(00)00078-8. [PubMed] [Cross Ref]
21. Froum SJ, Wallace S, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002;22:45–53. [PubMed]
22. Raghoebar GM, Schortinghuis J, Liem R, Ruben J, Wal J, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for the augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349–56. doi: 10.1111/j.1600-0501.2005.01115.x. [PubMed] [Cross Ref]
23. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–94. doi: 10.2165/00007256-200333050-00004. [PubMed] [Cross Ref]
24. Ranly D, Lohmann C, Andreacchio D, Boyan B, Schwartz Z. Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J Bone Joint Surg. 2007;89:139–46. doi: 10.2106/JBJS.F.00388. [PubMed] [Cross Ref]
25. Eppley B, Woodell J, Higgins J. Platelet Quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–7. doi: 10.1097/01.PRS.0000138251.07040.51. [PubMed] [Cross Ref]
26. Zehnder JL, Leung LLK. Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombim. Blood. 1990;76:2011–6. [PubMed]
27. Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–45. [PubMed]
28. Taylor M, Norman T, Clovis N, Blaha D. The response of rabbit patellar tendons after autologous blood injection. Med Sci Sports Exerc. 2002;34(1):70–3. doi: 10.1097/00005768-200201000-00012. [PubMed] [Cross Ref]
29. Berghoff W, Pietrzak W, Rhodes R. Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics. 2006;29(7):590–8. [PubMed]
30. Gardner MJ, Demetrakopoulos D, Klepchick P, Mooar P. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty: an analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2006;31:309–13. doi: 10.1007/s00264-006-0174-z. [PMC free article] [PubMed] [Cross Ref]
31. Everts P, Devilee R, Mahoney C, Eeftinck-Schattenenkerk M, Knape J, Zundert A. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:593–9. doi: 10.1111/j.1399-6576.2006.001005.x. [PubMed] [Cross Ref]
32. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–51. doi: 10.1016/j.transci.2004.01.004. [PubMed] [Cross Ref]
33. McAleer JP, Kaplan E, Persich G. Efficacy of concentrated autologous platelet-derived growth factors in chronic lower-extremity wounds. J Am Podiatr Med Assoc. 2006;96(6):482–8. [PubMed]
34. Ghandi A, Dumas C, O’Connor J, Parsons J, Lin S. The effects of local platelet rich plasma delivery on diabetic bone fracture healing. Bone. 2006;38:540–6. doi: 10.1016/j.bone.2005.10.019. [PubMed] [Cross Ref]
35. Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210–6. doi: 10.1016/S0736-0266(02)00066-9. [PubMed] [Cross Ref]
36. Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12(12):400–7. doi: 10.1007/s00586-003-0548-5. [PMC free article] [PubMed] [Cross Ref]
37. Carreon LY, Glassman SD, Anekstein Y, Puno RM. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30(9):E243–6. doi: 10.1097/01.brs.0000160846.85397.44. [PubMed] [Cross Ref]
38. Jenis LG, Banco RJ, Kwon B. A prospective study of Autologous Growth Factors (AGF) in lumbar interbody fusion. Spine J. 2006;6(1):14–20. doi: 10.1016/j.spinee.2005.08.014. [PubMed] [Cross Ref]
39. Castro FP., Jr Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17(5):380–4. doi: 10.1097/01.bsd.0000110342.54707.19. [PubMed] [Cross Ref]
40. Weiner BK, Walker M. Efficacy of autologous growth factors in autologous intertransverse fusions. Spine. 2003;28:1968–70. doi: 10.1097/01.BRS.0000083141.02027.48. [PubMed] [Cross Ref]
41. Lowery GL, Kulkarni S, Pennisi AE. Use of autologous growth factors in lumbar spine fusion. Bone. 1999;25:47S–50S. doi: 10.1016/S8756-3282(99)00132-5. [PubMed] [Cross Ref]
42. Chen W, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209(3):744–54. doi: 10.1002/jcp.20765. [PubMed] [Cross Ref]
43. Hunziker EB, Driesang IM, Morris EA. Clinical orthopaedics and related research. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res. 2001;391(Suppl):S171–81. doi: 10.1097/00003086-200110001-00017. [PubMed] [Cross Ref]
44. Nakagawa K, Sasho T, Arai M, Kitahara S, Ogino S, Wada Y, et al. Effects of autologous platelet-rich plasma on the metabolism of human articular chondrocytes. Chiba and Ichihara, Japan. Electronic poster presentation P181. International Cartilage Repair Society Meeting, Warsaw Poland, October 2007.
45. Kon E, Filardo G, Presti ML, Delcogliano M, Iacono F, Montaperto C, et al. Utilization of platelet-derived growth factors for the treatment of cartilage degenerative pathology. Bologna, Italy. Electronic poster presentation 29.3. International Cartilage Repair Society Meeting, Warsaw Poland, October 2007.
46. Anitua E, Sánchez M, Nurden AT, Zalduendo MM, Fuente M, Azofra J, et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology. 2007;46(12):1769–72. doi: 10.1093/rheumatology/kem234. [PubMed] [Cross Ref]
47. Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65(10):1951–7. doi: 10.1016/j.joms.2006.11.044. [PubMed] [Cross Ref]