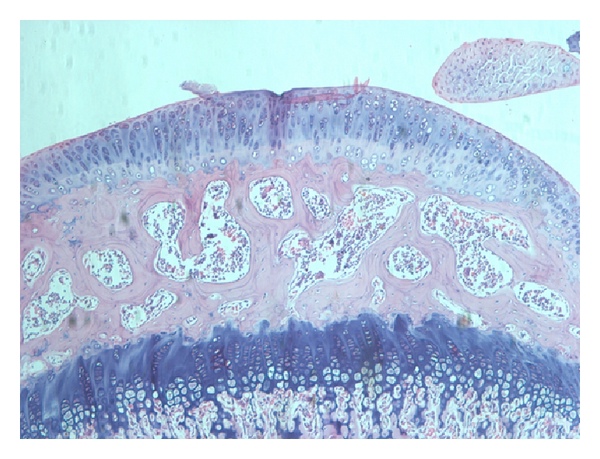
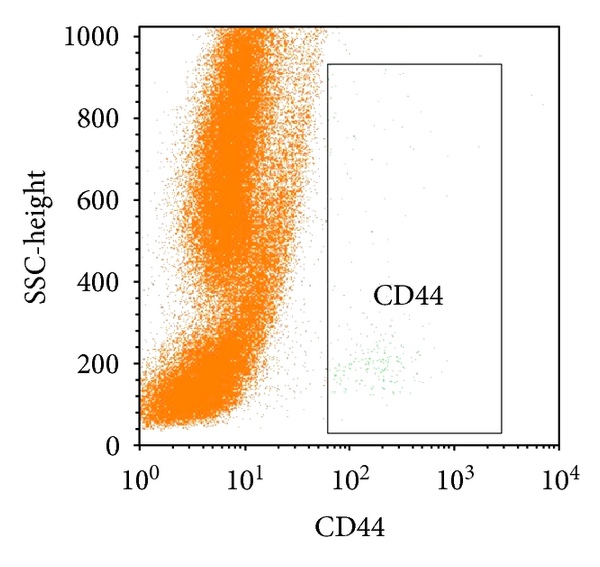
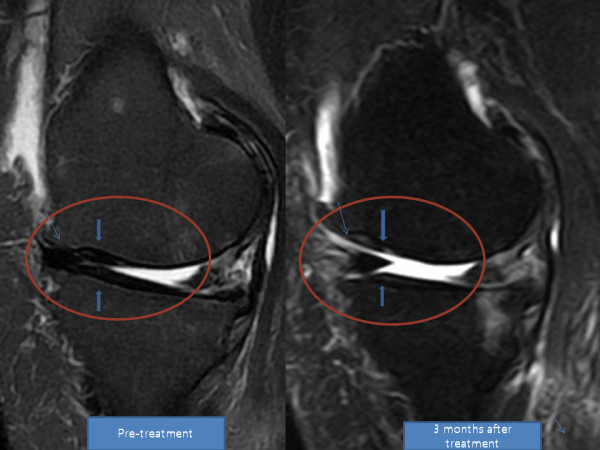
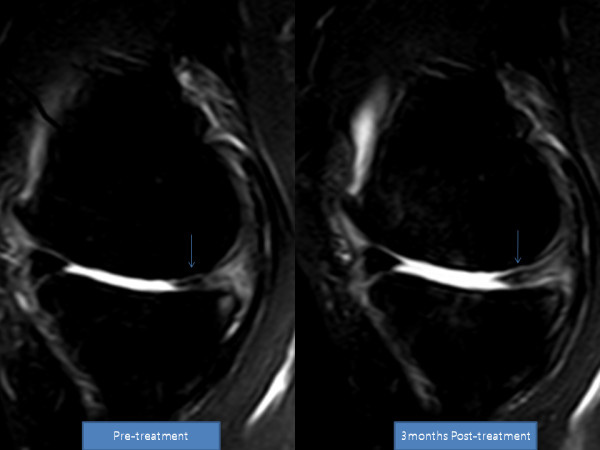
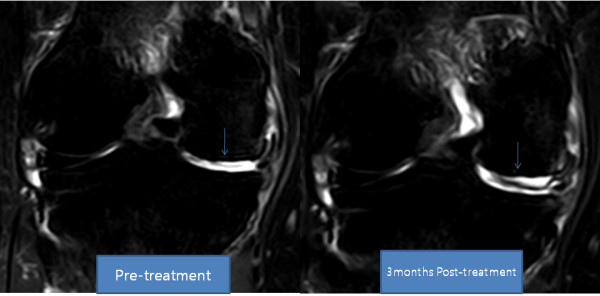
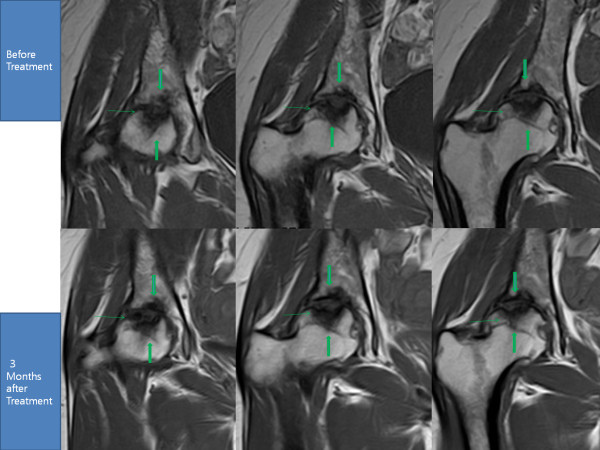
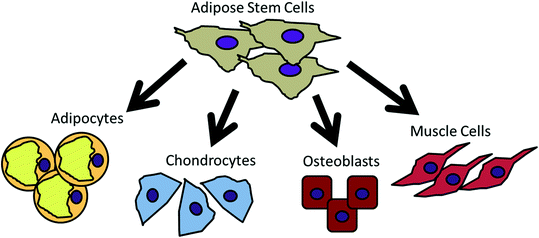
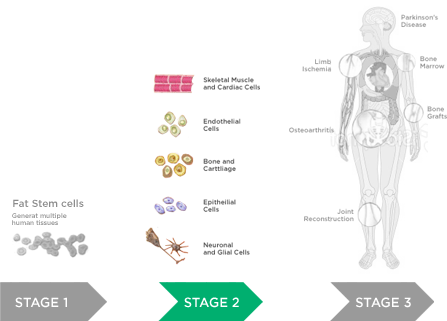
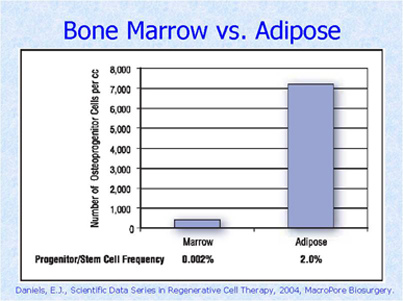
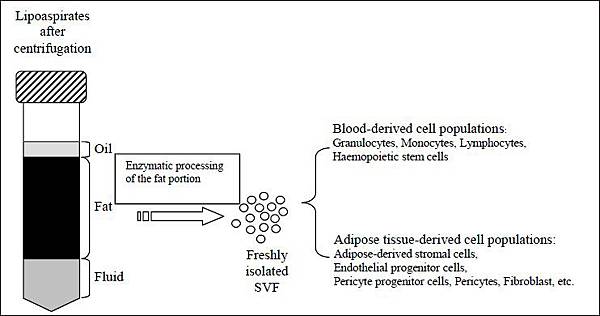
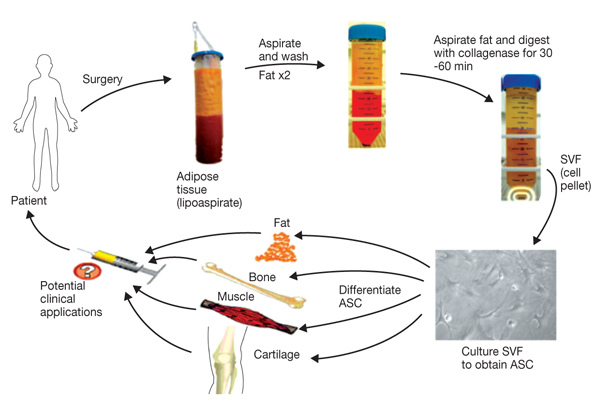
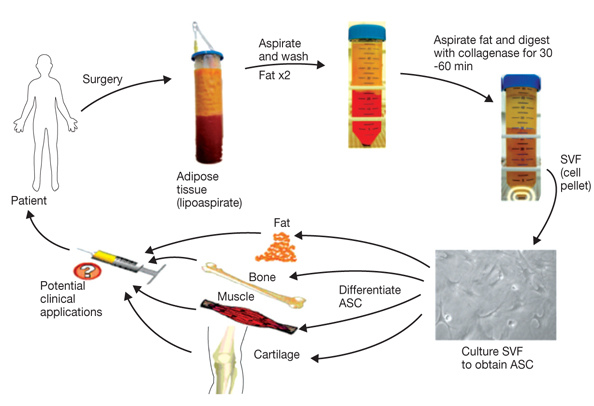
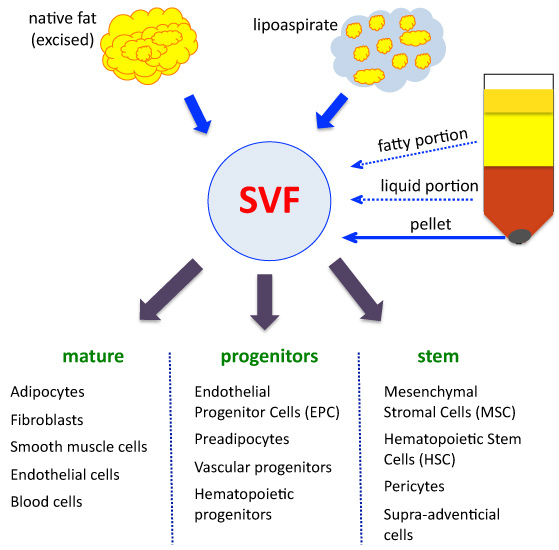
Transplantation of Non-expanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model(3)
References
- D. J. Prockop, “Marrow stromal cells as stem cells for non-hematopoietic tissues,” Science, vol. 276, pp. 71–74, 1997. View at Publisher · View at Google Scholar
- S. M. Phadnis, M. V. Joglekar, M. P. Dalvi et al., “Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo,” Cytotherapy, vol. 13, no. 3, pp. 279–293, 2011. View at Publisher · View at Google Scholar · View at Scopus
- B. T. Estes, B. O. Diekman, J. M. Gimble, and F. Guilak, “Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype,” Nature Protocols, vol. 5, no. 7, pp. 1294–1311, 2010. View at Publisher · View at Google Scholar · View at Scopus
- A. Reinisch, C. Bartmann, E. Rohde et al., “Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application,” Regenerative Medicine, vol. 2, no. 4, pp. 371–382, 2007. View at Publisher · View at Google Scholar · View at Scopus
- P. V. Phuc, T. H. Nhung, D. T. T. Loan, D. C. Chung, and P. K. Ngoc, “Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells,” In Vitro Cellular and Developmental Biology—Animal, vol. 47, no. 1, pp. 54–63, 2011. View at Publisher · View at Google Scholar · View at Scopus
- V. A. Farias, J. L. Linares-Fernández, J. L. Peñalver et al., “Human umbilical cord stromal stem cell express CD10 and exert contractile properties,” Placenta, vol. 32, pp. 86–95, 2011. View at Publisher · View at Google Scholar
- J. Peng, Y. Wang, L. Zhang et al., “Humanumbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro,” Brain Research Bulletin, vol. 84, pp. 235–243, 2011. View at Publisher · View at Google Scholar
- G. A. Pilz, C. Ulrich, M. Ruh et al., “Human term placenta-derived mesenchymal stromal cells are less prone to osteogenic differentiation than bone marrow-derived mesenchymal stromal cells,” Stem Cells and Development, vol. 20, no. 4, pp. 635–646, 2011. View at Publisher · View at Google Scholar · View at Scopus
- L. Spath, V. Rotilio, M. Alessandrini et al., “Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials,” Journal of Cellular and Molecular Medicine, vol. 14, pp. 1635–1644, 2010.
- A. M. Lubis and V. K. Lubis, “Adult bone marrow stem cells in cartilage therapy,” Acta Medica Indonesiana, vol. 44, pp. 62–68, 2012.
- C. Kasemkijwattana, S. Hongeng, S. Kesprayura, V. Rungsinaporn, K. Chaipinyo, and K. Chansiri, “Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report,” Journal of the Medical Association of Thailand, vol. 94, no. 3, pp. 395–400, 2011. View at Scopus
- F. Davatchi, B. S. Abdollahi, M. Mohyeddin, F. Shahram, and B. Nikbin, “Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients,” International Journal of Rheumatic Diseases, vol. 14, no. 2, pp. 211–215, 2011. View at Publisher · View at Google Scholar · View at Scopus
- D. Minteer, K. G. Marra, and J. P. Rubin, “Adipose-derived mesenchymal stem cells: biology and potential applications,” Advances in Biochemical Engineering/Biotechnology. In press.
- D. D. Frisbie, J. D. Kisiday, C. E. Kawcak, N. M. Werpy, and C. W. McIlwraith, “Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis,” Journal of Orthopaedic Research, vol. 27, no. 12, pp. 1675–1680, 2009. View at Publisher · View at Google Scholar · View at Scopus
- J. Pak, “Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series,” Journal of Medical Case Reports, vol. 5, p. 296, 2011. View at Publisher · View at Google Scholar · View at Scopus
- H. A. Wieland, M. Michaelis, B. J. Kirschbaum, and K. A. Rudolphi, “Osteoarthritis—an untreatable disease?” Nature Reviews Drug Discovery, vol. 4, pp. 331–344, 2005. View at Publisher · View at Google Scholar
- J. A. Buckwalter, C. Saltzman, and T. Brown, “The impact of osteoarthritis: implications for research,” Clinical Orthopaedics and Related Research, vol. 427, pp. S6–S15, 2004. View at Publisher · View at Google Scholar
- M. Dougados, “The role of anti-inflammatory drugs in the treatment of osteoarthritis: a European viewpoint,” Clinical and Experimental Rheumatology, vol. 19, pp. S9–S14, 2001. View at Publisher · View at Google Scholar
- T. Pincus, G. G. Koch, T. Sokka et al., “A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee,” Arthritis & Rheumatism, vol. 44, pp. 1587–1598, 2001. View at Publisher · View at Google Scholar
- S. Eyigor, S. Hepguler, M. Sezak, F. Öztop, and K. Capaci, “Effects of intra-articular hyaluronic acid and corticosteroid therapies on articular cartilage in experimental severe osteoarthritis,” Clinical and Experimental Rheumatology, vol. 24, no. 6, p. 724, 2006. View at Scopus
- T. Spaková, J. Rosocha, M. Lacko, D. Harvanová, and A. Gharaibeh, “Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid,” American Journal of Physical Medicine and Rehabilitation, vol. 91, no. 5, pp. 411–417, 2012.
- V. Karatosun, B. Unver, A. Ozden, Z. Ozay, and I. Gunal, “Intra-articular hyaluronic acid compared to exercise therapy in osteoarthritis of the ankle. A prospective randomized trial with long-term follow-up,” Clinical and Experimental Rheumatology, vol. 26, no. 2, pp. 288–294, 2008. View at Scopus
- J. P. Schroeppel, J. D. Crist, H. C. Anderson, and J. Wang, “Molecular regulation of articular chondrocyte function and its significance in osteoarthritis,” Histology and Histopathology, vol. 26, pp. 377–394, 2011.
- H. J. Harn, S. Z. Lin, S. H. Hung et al., “Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function,” Cell Transplantation. In press.
- J. H. Gu, Y. H. Ji, E. S. Dhong, D. H. Kim, and E. S. Yoon, “Transplantation of adipose derived stem cells for peripheral nerve regeneration in sciatic nerve defects of the rat,” Current Stem Cell Research & Therapy, vol. 7, no. 5, pp. 347–355, 2012. View at Publisher · View at Google Scholar
- N. Scuderi, S. Ceccarelli, M. G. Onesti et al., “Human adipose derived stem cells for cell based therapies in the treatment of systemic sclerosis,” Cell Transplantation. In press.
- M. Mazo, S. Hernández, J. J. Gavira et al., “Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical swine model of myocardial infarction,” Cell Transplantation. In press.
- R. Peçanha, L. L. Bagno, M. B. Ribeiro et al., “Adipose-derived stem-cell treatment of skeletal muscle injury,” The Journal of Bone & Joint Surgery, vol. 94, pp. 609–617, 2012.
- J. Xiao, C. Zhang, Y. Zhang et al., “Transplantation of adipose-derived mesenchymal stem cells into a murine model of passive chronic immune thrombocytopenia,” Transfusion, vol. 52, no. 12, pp. 2551–2558, 2012. View at Publisher · View at Google Scholar
- J. J. Yang, X. Yang, Z. Q. Liu et al., “Transplantation of adipose tissue-derived stem cells overexpressing heme oxygenase-1 improves functions and remodeling of infarcted myocardium in rabbits,” The Tohoku Journal of Experimental Medicine, vol. 226, pp. 231–241, 2012. View at Publisher · View at Google Scholar
- L. L. Black, J. Gaynor, C. Adams et al., “Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs,” Veterinary Therapeutics, vol. 9, no. 3, pp. 192–200, 2008. View at Scopus
- L. L. Black, J. Gaynor, D. Gahring et al., “Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial,” Veterinary Therapeutics, vol. 8, no. 4, pp. 272–284, 2007. View at Scopus
- A. Guercio, P. Di Marco, S. Casella et al., “Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints,” Cell Biology International, vol. 36, pp. 189–194, 2012. View at Publisher · View at Google Scholar
- F. S. Toghraie, N. Chenari, M. A. Gholipour et al., “Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit,” Knee, vol. 18, no. 2, pp. 71–75, 2011. View at Publisher · View at Google Scholar · View at Scopus
- J. M. Lee and G. I. Im, “SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat,” Biomaterials, vol. 33, pp. 2016–2024, 2012. View at Publisher · View at Google Scholar
- M. C. ter Huurne, P. L. E. M. van Lent, A. B. Blom, et al., “A single injection of adipose-derived stem cells protects against cartilage damage and lowers synovial activation in experimental osteoarthritis,” Arthritis & Rheumatism, vol. 63, p. 1784, 2011.
- J. M. Gimble and F. Guilak, “Differentiation potential of adipose derived adult stem cell (ADAS) cells,” Current Topics in Developmental Biology, vol. 58, pp. 137–160, 2003. View at Publisher · View at Google Scholar
- J. M. Murphy, D. J. Fink, E. B. Hunziker, and F. P. Barry, “Stem cell therapy in a caprine model of osteoarthritis,” Arthritis & Rheumatism, vol. 48, pp. 3464–3474, 2003. View at Publisher · View at Google Scholar
- P. A. Zuk, M. Zhu, H. Mizuno et al., “Multilineage cells from human adipose tissue: implications for cell-based therapies,” Tissue Engineering, vol. 7, no. 2, pp. 211–228, 2001. View at Publisher · View at Google Scholar · View at Scopus
- Y. D. C. Halvorsen, D. Franklin, A. L. Bond et al., “Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells,” Tissue Engineering, vol. 7, no. 6, pp. 729–741, 2001. View at Publisher · View at Google Scholar · View at Scopus
- H. Mizuno, P. A. Zuk, M. Zhu, H. P. Lorenz, P. Benhaim, and M. H. Hedrick, “Myogenic differentiation by human processed lipoaspirate cells,” Plastic and Reconstructive Surgery, vol. 109, pp. 199–209, 2002. View at Publisher · View at Google Scholar
- W. Wagner, F. Wein, A. Seckinger et al., “Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood,” Experimental Hematology, vol. 33, no. 11, pp. 1402–1416, 2005. View at Publisher · View at Google Scholar · View at Scopus
- S. Kern, H. Eichler, J. Stoeve, H. Klüter, and K. Bieback, “Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue,” Stem Cells, vol. 24, no. 5, pp. 1294–1301, 2006. View at Publisher · View at Google Scholar · View at Scopus
- K. M. Safford, K. C. Hicok, S. D. Safford et al., “Neurogenic differentiation of murine and human adipose-derived stromal cells,” Biochemical and Biophysical Research Communications, vol. 294, no. 2, pp. 371–379, 2002. View at Publisher · View at Google Scholar · View at Scopus
- R. Izadpanah, C. Trygg, B. Patel et al., “Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue,” Journal of Cellular Biochemistry, vol. 99, no. 5, pp. 1285–1297, 2006. View at Publisher · View at Google Scholar · View at Scopus
- A. N. Patel, J. Yochman, V. Vargas, and D. A. Bull, “Putative population of adipose derived stem cells isolated from mediastinal tissue during cardiac surgery,” Cell Transplantation. In press. View at Publisher · View at Google Scholar
- G. Musumeci, D. Lo Furno, C. Loreto et al., “Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin,” Experimental Biology and Medicine, vol. 236, pp. 1333–1341, 2011. View at Publisher · View at Google Scholar
- V. Zachar, J. G. Rasmussen, and T. Fink, “Isolation and growth of adipose tissue-derived stem cells,” Methods in Molecular Biology, vol. 698, pp. 37–49, 2011. View at Publisher · View at Google Scholar · View at Scopus
- C. K. Rebelatto, A. M. Aguiar, M. P. Moretão et al., “Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue,” Experimental Biology and Medicine, vol. 233, no. 7, pp. 901–913, 2008. View at Publisher · View at Google Scholar · View at Scopus
- I. S. Blande, V. Bassaneze, C. Lavini-Ramos et al., “Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate,” Transfusion, vol. 49, no. 12, pp. 2680–2685, 2009. View at Publisher · View at Google Scholar · View at Scopus
- C. Dromard, P. Bourin, M. André, S. De Barros, L. Casteilla, and V. Planat-Benard, “Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres,” Experimental Cell Research, vol. 317, no. 6, pp. 770–780, 2011. View at Publisher · View at Google Scholar · View at Scopus
- D. T. B. Shih, J. C. Chen, W. Y. Chen, Y. P. Kuo, C. Y. Su, and T. Burnouf, “Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate,” Transfusion, vol. 51, no. 4, pp. 770–778, 2011. View at Publisher · View at Google Scholar · View at Scopus
- M. Dominici, K. Le Blanc, I. Mueller et al., “Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement,” Cytotherapy, vol. 8, no. 4, pp. 315–317, 2006. View at Publisher · View at Google Scholar · View at Scopus
- H. Niwa, J. Miyazaki, and A. G. Smith, “Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells,” Nature Genetics, vol. 24, pp. 372–376, 2000. View at Publisher · View at Google Scholar
- K. Mitsui, Y. Tokuzawa, H. Itoh et al., “The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells,” Cell, vol. 113, no. 5, pp. 631–642, 2003. View at Publisher · View at Google Scholar · View at Scopus
- K. Hochedlinger, Y. Yamada, C. Beard, and R. Jaenisch, “Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues,” Cell, vol. 121, no. 3, pp. 465–477, 2005. View at Publisher · View at Google Scholar · View at Scopus
- S. H. Chiou, C. C. Yu, C. Y. Huang et al., “Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma,” Clinical Cancer Research, vol. 14, no. 13, pp. 4085–4095, 2008. View at Publisher · View at Google Scholar · View at Scopus
- Y. C. Chen, H. S. Hsu, Y. W. Chen et al., “Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells,” PLoS One, vol. 3, article e2637, 2008.
- C. Liu, X. Cao, Y. Zhang et al., “Co-expression of Oct-4 and Nestin in human breast cancers,” Molecular Biology Reports, vol. 39, pp. 5875–5881, 2012. View at Publisher · View at Google Scholar
- Y. Guo, S. Liu, P. Wang et al., “Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas,” Histopathology, vol. 59, pp. 763–775, 2011. View at Publisher · View at Google Scholar
- J. T. Oliveira, L. S. Gardel, T. Rada, L. Martins, M. E. Gomes, and R. L. Reis, “Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects,” Journal of Orthopaedic Research, vol. 28, no. 9, pp. 1193–1199, 2010. View at Publisher · View at Google Scholar · View at Scopus
- J. L. Dragoo, G. Carlson, F. McCormick et al., “Healing full-thickness cartilage defects using adipose-derived stem cells,” Tissue Engineering, vol. 13, no. 7, pp. 1615–1621, 2007. View at Publisher · View at Google Scholar · View at Scopus
- P. F. Caimi, J. Reese, Z. Lee, and H. M. Lazarus, “Emerging therapeutic approaches for multipotent mesenchymal stromal cells,” Current Opinion in Hematology, vol. 17, no. 6, pp. 505–513, 2010. View at Publisher · View at Google Scholar · View at Scopus
- N. G. Singer and A. I. Caplan, “Mesenchymal stem cells: mechanisms of inflammation,” Annual Review of Pathology, vol. 6, pp. 457–478, 2011. View at Publisher · View at Google Scholar
- O. Ringdén, M. Uzunel, I. Rasmusson et al., “Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease,” Transplantation, vol. 81, no. 10, pp. 1390–1397, 2006. View at Publisher · View at Google Scholar · View at Scopus
- B. Fang, Y. Song, L. Liao, Y. Zhang, and R. C. Zhao, “Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease,” Transplantation Proceedings, vol. 39, no. 10, pp. 3358–3362, 2007. View at Publisher · View at Google Scholar · View at Scopus
- B. Fang, Y. Song, R. C. Zhao, Q. Han, and Q. Lin, “Using human adipose tissue-derived mesenchymal stem cells as salvage therapy for hepatic graft-versus-host disease resembling acute hepatitis,” Transplantation Proceedings, vol. 39, no. 5, pp. 1710–1713, 2007. View at Publisher · View at Google Scholar · View at Scopus
- P. Borrione, A. D. Gianfrancesco, M. T. Pereira, and F. Pigozzi, “Platelet-rich plasma in muscle healing,” American Journal of Physical Medicine & Rehabilitation, vol. 89, pp. 854–861, 2010. View at Publisher · View at Google Scholar
- W. Yu, J. Wang, and J. Yin, “Platelet-rich plasma: a promising product for treatment of peripheral nerve regeneration after nerve injury,” International Journal of Neuroscience, vol. 121, pp. 176–180, 2011. View at Publisher · View at Google Scholar
- S. Sampson, M. Reed, H. Silvers, M. Meng, and B. Mandelbaum, “Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study,” American Journal of Physical Medicine & Rehabilitation, vol. 89, no. 12, pp. 961–969, 2010. View at Scopus
- G. Filardo, E. Kon, R. Buda et al., “Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis,” Knee Surgery, Sports Traumatology, Arthroscopy, vol. 19, no. 4, pp. 528–535, 2011. View at Publisher · View at Google Scholar · View at Scopus