

Avoiding Malar Edema During Midface/Cheek Augmentation with Dermal Fillers(2)
Fillers, alone or in conjunction with facial surgery, can restore facial harmony, balance, and beauty. Nevertheless, treatment of this area is not without its complications. Bruising, erythema, pain, Infection, Skin necrosis, Over- and Under- correction, and Infraorbital nerve injury resulting in numbness and dysesthesia have been reported, regardless of the filler type (hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid) used. Nodules, Lumps, visible material, and generalized and Malar edema may also occur. Malar edema is a particularly significant adverse event because it is disfiguring, poorly tolerated by patients, can persist for months, and responds minimally, if at all, to treatment.
Anatomic Basis for Malar Edema
Malar edema is an adverse event arising from filler injections of the central midface to correct the Infraorbital hollow and Tear trough. It occurred in two patients treated by the author after placing Calcium hydroxylapatite (CaHA, Radiesse®, Merz Aesthetics, San Mateo, California) in a retrograde linear threading and fanning technique from multiple access points. The edema was long lived—6 to 8 months—and only minimally responsive to massage, head elevation, taping, salt avoidance, methylprednisolone, and conservative intralesional steroid injections. Although these two cases involved CaHA, malar edema arising from injections with hyaluronic acid (HA) has also been seen in clinical practice.
The phenomenon of malar edema can be explained by an understanding of the anatomy of the lower eyelid. Pessa and Garza6,7 reported their findings after performing 18 fresh cadaver dissections. Using dye injections, histological evaluation, and gross anatomical dissection, they identified a Fascial structure of the lower eyelid and cheek that they called the Malar septum. It originates from the Orbital rim periosteum at the Arcus marginalis superiorly and inserts into the Cheek skin 2.5 to 3cm inferior to the Lateral canthus. It divides the Suborbicularis oculi fat (SOOF) into Superior and Inferior components. The Inferior component is confluent with the Cheek fat and the Superior component contributes to the Malar mounds. At the level of the inferior border of the orbicularis oculi, the malar septum fuses with the fibrous septa of the superficial cheek fat and dermis.
The authors stated that the Malar septum is a relatively impermeable barrier that allows tissue edema and hemoglobin to accumulate superior to its Cutaneous insertion, and thus defines the lower anatomical boundary of several clinical entities : Malar edema, Malar mounds, Festoons, and Periorbital ecchymosis. Its anatomy is consistent from person to person regardless of age.
Transcutaneous Preperiosteal Injection Using Limited Puncture Sites
The area bounded by the Lower eyelid margin superiorly, the Medial canthus medially, the Lateral canthus laterally, and the Submalar region inferiorly is the least forgiving and most prone to adverse events. Injected filler Superficial to the malar septum may serve to Augment the impermeable barrier of the Malar septum, further impeding lymphatic drainage resulting in fluid accumulation and prolonged edema. Fillers may also cause edema by Direct pressure on the lymphatics when Volumes are too large whether they are superficial or deep to the septum. In addition, the greater a filler's Elasticity or Elastic modulus (G')—lifting capacity—the more likely it is to compress the lymphatic flow, resulting in edema. Malar edema is likely related to the Volume of injectate, the filler's Physical characteristics, its Depth of injection, and the patient's Propensity toward the problem.
Any filler injected within the boundaries of the malar septum should be placed immediately onto Periosteum (Pre-periosteally). In addition to avoiding malar edema, placing Small boluses of filler directly on Bone has the additional advantage of avoiding lumps, nodules, and visible material. The result is more natural and aesthetically pleasing because it is an augmentation of the underlying skeletal structure, resulting in an expansion and elevation of the overlying soft tissue envelope. Since the material is placed in an Avascular space, there is less bruising and lower embolic potential. Preperiosteal small bolus technique can be accomplished using either an Intraoral or Transcutaneous approach. The author prefers a transcutaneous approach because it is less technically demanding, easier to teach, has less risk of infraorbital nerve injury and in theory has less risk of infection and biofilm creation. Another approach would be the use of a Nonparticulate, Monophasic, less refractive HA capable of being placed in the Subdermal plane without being visible or causing a Tyndall effect. This would allow correction of the tear trough and infraorbital hollow without compressing the deeper lymphatic structures.
Technique. A careful examination of the patient is made, being observant for any evidence of existing malar edema or malar bags. Inquiry about a history of cheek edema after excessive salt or alcohol intake or upon awakening is followed by a discussion to assure that the patient is in agreement with the treatment plan. In a patient who is unsure, a “Trial run”, wherein Lidocaine is injected to simulate her postinjection appearance, can be performed. Preinjection photographs are taken. Small volume, bilateral infraorbital nerve blocks are placed with 2% lidocaine no more than 0.2mL. The filler is mixed with 2% lidocaine with 1:100,000 epinephrine.8 A volume of 0.2mL of 2% Lidocaine with Epinephrine is sterilely mixed with a 1.5mL syringe of CaHA and 0.08mL of 2% Lidocaine with Epinephrine with a 0.8mL syringe of hyaluronic acid Juvederm Ultra (HA-JU, Allergan).
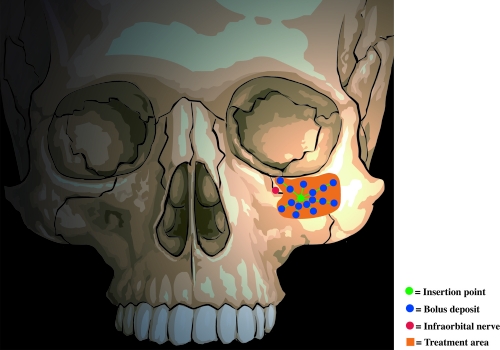
The first injection is placed Medial to the Infraorbital nerve, entering perpendicular to the skin approximately 1cm Beneath the Inferior orbital rim (Figure 2). The needle is then “walked” medially toward the medial canthus, depositing 0.05mL aliquots. Additional deposits are placed close to the orbital rim as well as laterally and inferiorly. The Nondominant index finger is used to establish the Inferior orbital rim location so as to prevent deposition of material into the orbital area. If the needle is in contact with the bone at the time of extrusion of filler, then the material will not be deposited into the infraorbital foramen or within the infraorbital nerve. The second injection is Lateral to the infraorbital nerve; the third is at the Malar eminence (Figures 3 and and44).
Figure 2
First injection is placed medial to the infraorbital nerve, entering perpendicular to the skin one cm beneath the inferior orbital rim. The needle is then “walked” medially toward the medial canthus, depositing 0.05 mL aliquots.
Figure 3
The second injection is lateral to the infraorbital nerve.
Figure 4
The third injection is at the malar eminence.



 留言列表
留言列表
