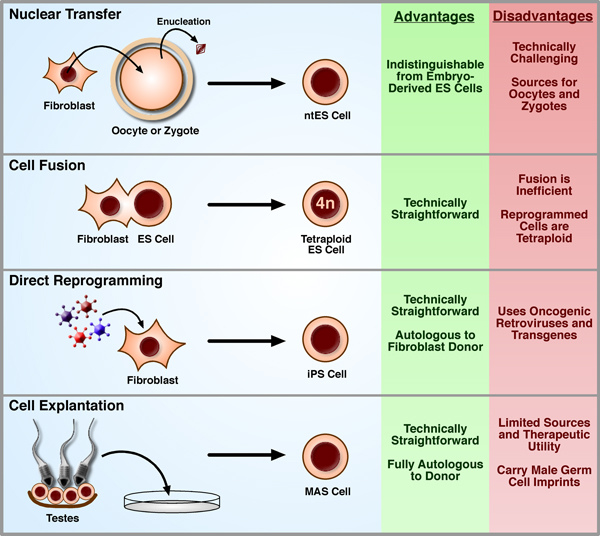
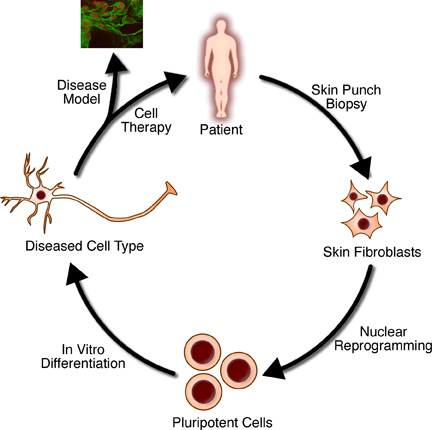
Inducing pluripotency(3)
Four techniques for restoring developmental potential to a somatic nucleus have been described in the literature. In nuclear transfer, the genetic material of an oocyte or zygote is replaced with that of a differentiated cell such as a fibroblast. Following development to the blastocyst stage, Pluripotent ntES cells can be derived as from fertilized embryos. In cellular fusion, hybridization between ES cells and somatic cells yields Tetraploid ES cell lines. In direct reprogramming, the retroviral-mediated introduction of a small number of transcription factors is sufficient to confer a pluripotent phenotype. Finally, explantation of testes tissue from neonatal and adult mice into appropriate culture conditions has been shown to result in the production of Multipotent adult spermatagonial(MAS)cells.
2. Nuclear transfer
Building on the early work of developmental biology pioneers such as Spemann(Gurdon and Byrne, 2003; Spemann, 1938), Nuclear transfer(NT;also commonly called Somatic cell nuclear transfer,SCNT)experiments were first devised in the 1950s as a means to investigate the constancy of the genome: that is, whether cells maintained the full complement of genomic information as they became more differentiated. At the time, many believed that each cell fate decision during development involved the progressive loss of genes that would not be used by the more differentiated progeny. For instance, ectoderm precursors eliminating all endoderm- and mesoderm-specific genes, then eliminating skin-specific genes as the decision to become a neural precursor is made, eventually yielding a specific type of neuron with a minimal genome containing only the genes which would actually be transcribed. NT experiments in the frogs Rana pipiens by Briggs and King(Briggs and King, 1952)and Xenopus laevis by Gurdon(Gurdon et al., 1958;Gurdon et al., 1975)indicated that, although the generation of clones became less and less efficient as the developmental age of the donor nucleus increased, it was possible to obtain heartbeat-stage tadpoles from terminally differentiated adult cells. It was not until the more recent cloning of Dolly the sheep(Wilmut et al., 1997), however, that researchers succeeded in using a cell from an adult animal to generate another healthy, fertile adult, thereby demonstrating that the nuclei of at least some cells in the adult maintained a full developmental capacity. While an important finding, many speculated that the rare cloned adult animals could have arisen from the nucleus of an equally rare somatic stem cell. Definitive demonstration that terminally differentiated adult nuclei maintain full developmental capacity was later achieved in mouse studies using Mature lymphocytes(Hochedlinger and Jaenisch, 2002;Inoue et al., 2005)and Olfactory neurons(Eggan et al., 2004)as NT donors.
Dolly's birth and an initial report of the derivation of human ES cells from discarded in vitro fertilization(IVF)embryos shortly thereafter(Thomson et al., 1998)led to wide speculation in both the media and scientific community about the possibility of therapeutic cloning. That is, performing Nuclear transfer with a patient's Somatic cells to generate a Preimplantation embryo from which patient-specific ES cells might be derived for use in personalized regenerative medicine. With this motivation, several studies in mouse have sought to compare the properties of SCNT-derived ES(ntES)cells with those derived from naturally fertilized embryos(Brambrink et al., 2006; Wakayama et al., 2006), as well as show proof-of-principle demonstrations of these cells’ utility in regenerative medicine(Barberi et al., 2003; Rideout et al., 2002; Tabar et al., 2008). The equivalence of ntES cells to ES cells from fertilized embryos was of particular concern because of the low efficiencies and common health defects associated with animals brought to term after reproductive cloning(Eggan et al., 2001; Gurdon and Byrne, 2003; Humpherys et al., 2001). Despite these defects, however, two studies evaluating the transcriptional profiles, DNA methylation patterns, and in vitro differentiation capacity found that mouse ntES cell lines were identical in all regards to genetically-matched control ES cell lines derived after fertilization(Brambrink et al., 2006; Wakayama et al., 2006). These reassuring results, paired with reports using ntES cells to treat mouse models of both severe compromised immunodeficiency(Rideout et al., 2002)and Parkinson's diseases(Barberi et al., 2003;Tabar et al., 2008)after in vitro genetic manipulations and differentiation into the required cell types, generated immense hope that applications to human diseases were immediately on the horizon.
While ntES cells hold great promise for the field of regenerative medicine, the technique has several significant drawbacks that hinder its potential for widespread application to medicine or even to the study of the process of nuclear reprogramming. Obstacles associated with the requirement for a scarce and politically charged cell type, human oocytes, as a recipient cytoplasm are only amplified by the extreme technical challenge of a method plagued with inherent inefficiencies. Moreover, the experimental requirements for successful nuclear transfer in primates appear to be considerably different than other species, with attempts to apply the methods and techniques from mouse directly to human thus far yielding nothing more than false starts(Kennedy, 2006)and the rare report of NT-derived blastocysts but with no ES cell lines derived from them(French et al., 2008; Stojkovic et al., 2005). Likewise, only very recently has successful nuclear transfer in monkeys been reported(Byrne et al., 2007), but the technical refinements developed here have failed to immediately translate into advances with human cells.
A particularly severe limitation of human therapeutic cloning which has significantly hindered researchers’ capacity to develop the technique is the difficulty in obtaining donated oocytes. A 2007 study in mouse suggests that there may be alternative sources for the recipient cytoplast(Egli et al., 2007). While early mouse NT studies in which the enucleated interphase zygote was used as a recipient lead to the conclusion that reprogramming capacity was lost following fertilization(McGrath and Solter, 1984;Wakayama et al., 2000), Egli and coworkers demonstrated that by removing the chromatin from a zygote arrested in metaphase just prior to the first cell division and introducing the chromatin from a metaphase-arrested somatic cell into this cytoplast, reprogramming could occur with success rates comparable to NT into the mature oocyte(which is naturally arrested in metaphase;see Figure 3). The authors reasoned, therefore, that nuclear factors, trapped in the interphase nucleus but present in the cytoplasm during metaphase due to nuclear envelope breakdown, were necessary for reprogramming to occur. Not only might the Metaphase zygote therefore serve as an appropriate recipient for NT, but this result suggests that other Cleavage-stage blastomeres, arrested in mitosis, may be as well. These findings both shed some mechanistic light on the process of reprogramming and open the door to using a wider range of materials for human NT experiments. Although on the surface this study holds great promise for human NT experiments, it is unfortunately not common IVF practice to freeze or discard zygotes or early cleavage-stage embryos, as embryo quality can be difficult to assess so soon following fertilization(Salumets et al., 2001). Of particular interest, however, Egli et al. further demonstrated that Polyspermic zygotes(that is, embryos fertilized with multiple sperm)could be used for successful nuclear transfer. Multiple fertilization is relatively common in IVF(roughly 3–5% of zygotes)and these embryos, which have no clinical use, are routinely discarded(Anon, 2004;van der Ven et al., 1985). As such, discarded polyspermic IVF embryos may present a valuable new avenue towards success in human nuclear transfer.
Figure 3. Reprogramming capacity in NT depends on cell-cycle status, as demonstrated by Egli et al.(Egli et al., 2007).
Development fails after replacing the interphase nucleus of either a germinal-vesicle stage oocyte or pronuclear zygote with somatic chromatin. However, Transfer of somatic chromatin into either the MII-arrested oocyte or a Zygote arrested with a drug in the first mitosis allows for the generation of cloned mice and ntES cells.
Despite the challenges and limited achievements in human, NT remains the “Gold standard” in Nuclear reprogramming with clear demonstrations of the production of both Healthy clones and Pluripotent stem cells identical to those derived from fertilized embryos. Nevertheless, the development of more robust and technically simple reprogramming methods(discussed below)appears to be at hand, leading some authors to write obituaries for SCNT(Cibelli, 2007;Highfield, 2007). Whether these eulogies are premature or whether the quality of pluripotent cells generated by newer techniques will prove to be as high as ntES is presently an area of intense investigation.