




Carpal tunnel release using the MANOS CTR system:preliminary results in 52 patients.
McCormack B1, Bowen W, Gunther S, Linthicum J, Kaplan M, Eyster E.
J Hand Surg Am. 2012 Apr;37(4):689-94. doi: 10.1016/j.jhsa.2011.12.033. Epub 2012 Feb 24.
Abstract
PURPOSE
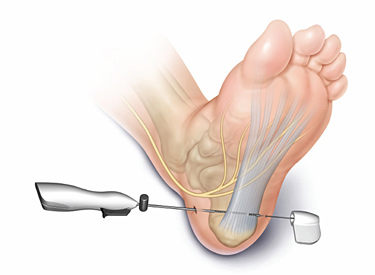
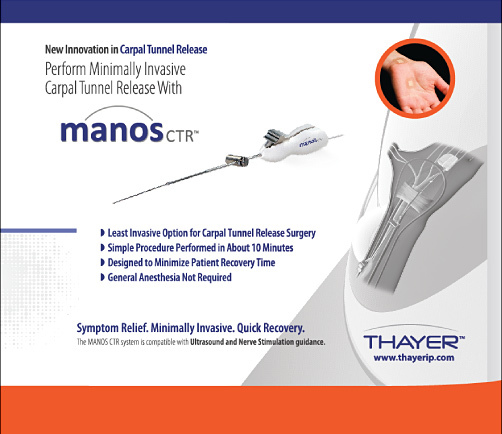
To describe a carpal tunnel release technique using the MANOS Carpal Tunnel Release device, with preliminary results in 52 patients.
METHODS
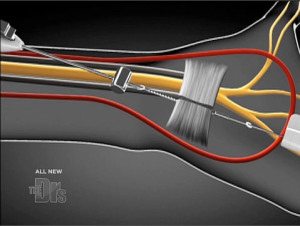
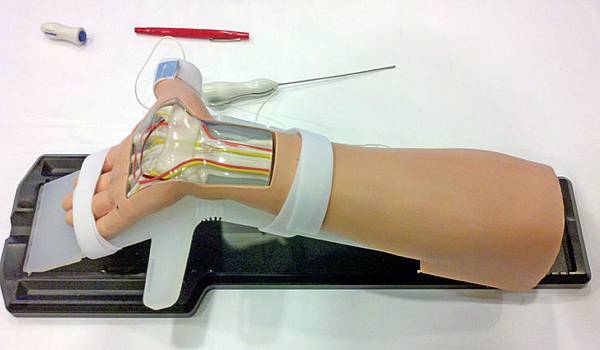
The MANOS Carpal Tunnel Release device is a blade that divides the transverse carpal ligament using wrist and palm skin punctures. The awake patient provides feedback as the surgeon navigates a 2.1-mm-diameter blunt probe across the undersurface of the ligament from a wrist incision with standard disposable nerve stimulator monitoring. The leading tip of the blunt probe is uninsulated and conducts 2 mA. The surgeon converts the blunt insulated probe into an uninsulated blade by advancing a 0.9-mm needle through the palm with a thumb-activated deployment feature. The surgeon saws the ligament through the 2 skin punctures. We used a validated outcome questionnaire to assess postoperative symptoms at 3 months.
RESULTS
Symptom severity and functional status scores compare favorably with literature controls for open and endoscopic surgery at 3 months. One patient required reoperation for incomplete release. There were no tendon or nerve injuries.
CONCLUSIONS
Preliminary results suggest the MANOS Carpal Tunnel Release device to be safe and effective.
TYPE OF STUDY/LEVEL OF EVIDENCE:Therapeutic IV.



 留言列表
留言列表
