
Cancer stem cells(CSCs,癌症幹細胞)and Stemline therapy or CSC-targeted cancer therapy
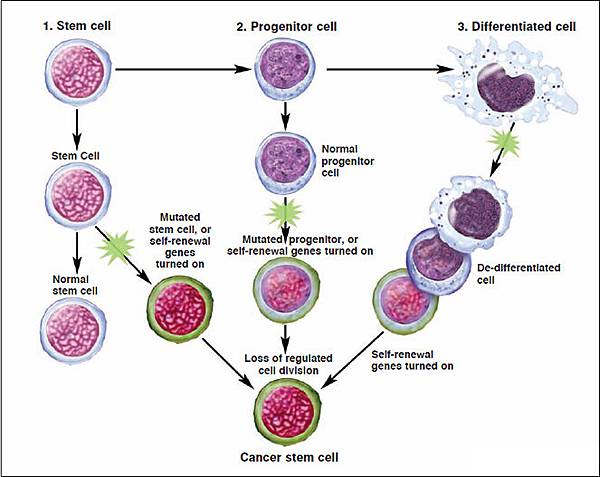
Cancer stem cells(CSCs)are cancer cells found within tumors or hematological cancers that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample.
CSCs are therefore tumorigenic(tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells.
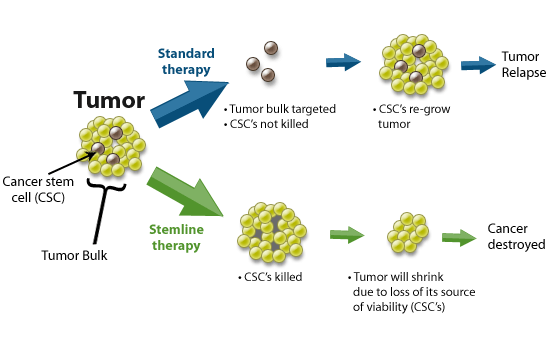
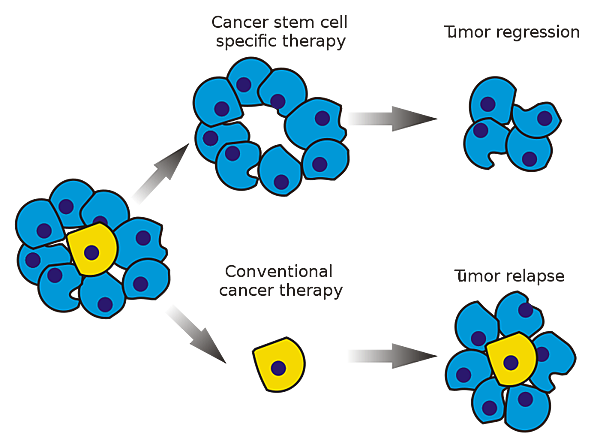
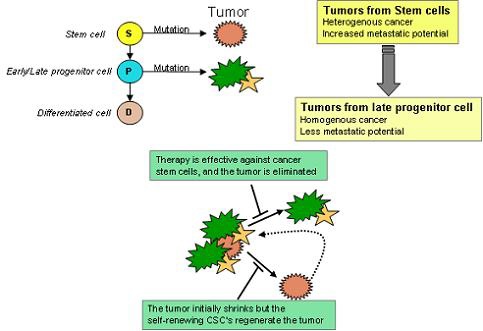
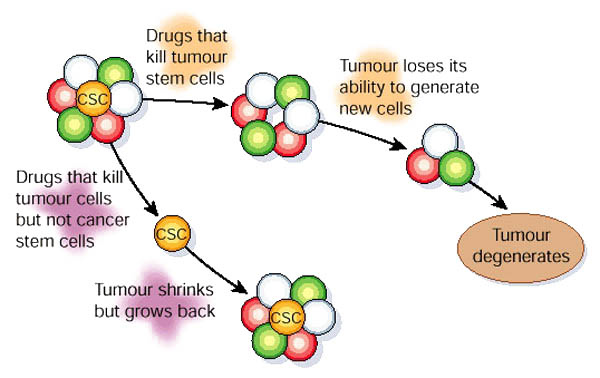
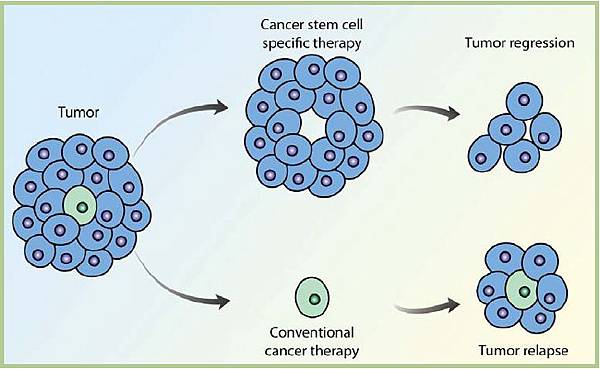
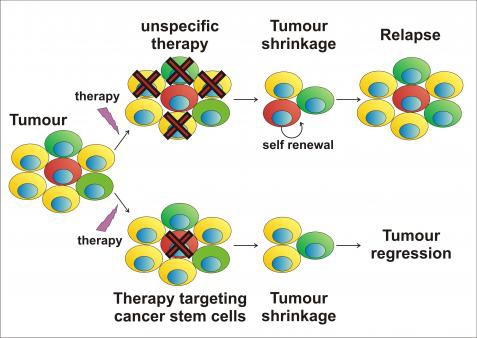
CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease.
Existing cancer treatments have mostly been developed based on animal models, where therapies able to promote tumor shrinkage were deemed effective. However, animals could not provide a complete model of human disease. In particular, in mice, whose life spans do not exceed two years, tumor relapse is exceptionally difficult to study.
The efficacy of cancer treatments is, in the initial stages of testing, often measured by the ablation fraction of tumor mass(fractional kill). As CSCs would form a very small proportion of the tumor, this may not necessarily select for drugs that act specifically on the stem cells. The theory suggests that Conventional chemotherapies kill Differentiated or Differentiating cells, which form the bulk of the tumor but are unable to generate new cells. A population of CSCs, which gave rise to it, could remain untouched and cause a relapse of the disease.
癌症幹細胞(Cancer Stem Cell,CSC),又稱癌幹細胞、腫瘤幹細胞,是指具有幹細胞(Stem cell)性質的癌細胞,也就是具有「自我複製」(self-renewal)以及「具有多細胞分化」(differentiation)等能力。通常這類的細胞被認為有形成腫瘤,發展成癌症的潛力,特別是隨著癌症轉移出去後,產生新型癌症的來源。
腫瘤內許多的細胞,其實只有一群細胞具有永生不死、持續分裂、分化的能力,而這些細胞,就稱為癌症幹細胞。
癌症幹細胞被認為是造成癌症轉移、復發,或是腫瘤對於化療、放射性療法產生抗性的原因之一。目前針對這個理論,學者希望研究出針對癌症幹細胞的療法,能夠專一性地殺死癌症幹細胞,以降低腫瘤產生抗藥性或是轉移的現象。
目前的癌症療法都是基於動物實驗的結果,目前的認知是只要癌症療法能將動物身上的腫瘤有效地縮小,就會被認為有其療效,然而要將實驗結果對照到人體醫療上仍有相當大的差距。比如說,以小鼠為例,生活週期很難超過兩年,如此短的生活週期無法提供癌症復發的動物模型可供研究。
而癌症幹細胞的理論認為,因為現行的治療都無法專一性的針對癌症幹細胞,而癌症幹細胞在腫瘤裡其實只佔一小部分,只要化療、放射性療法沒有殺死所有的癌症幹細胞,就會有抗藥性或是復發的風險出現。
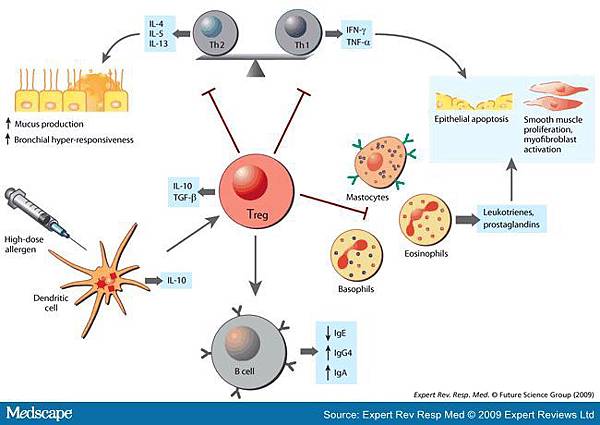
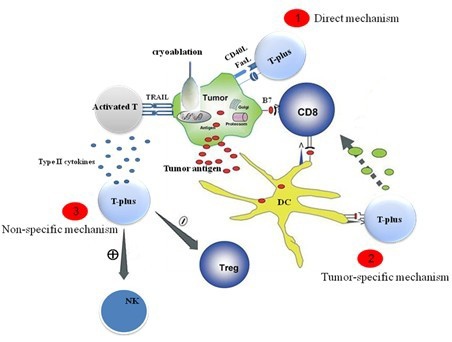
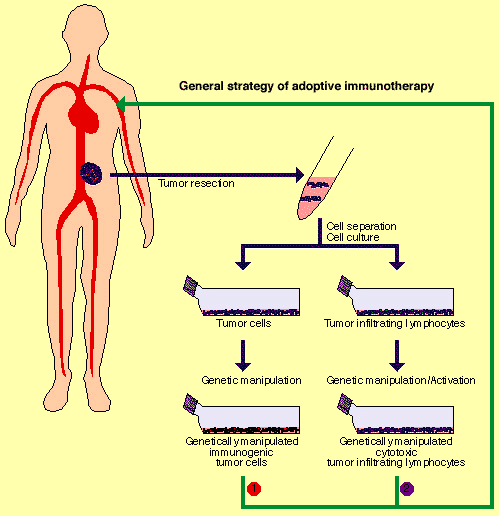
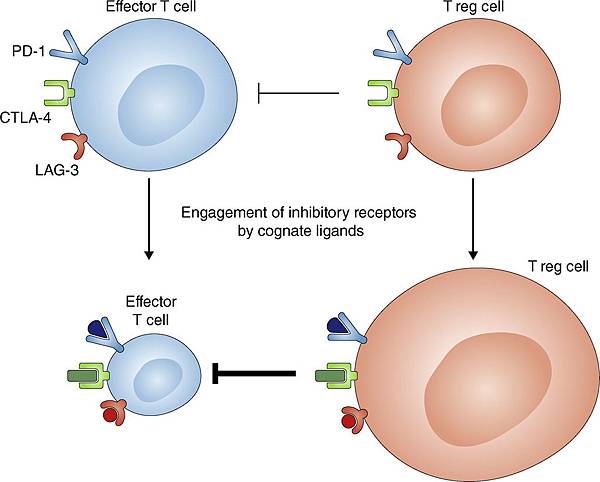
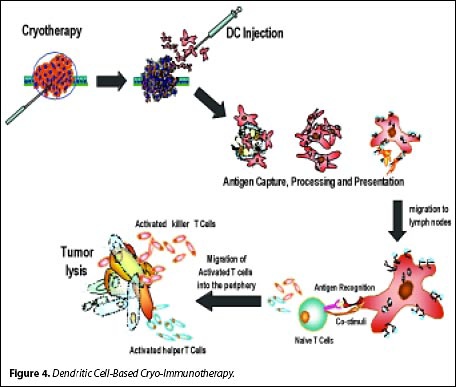

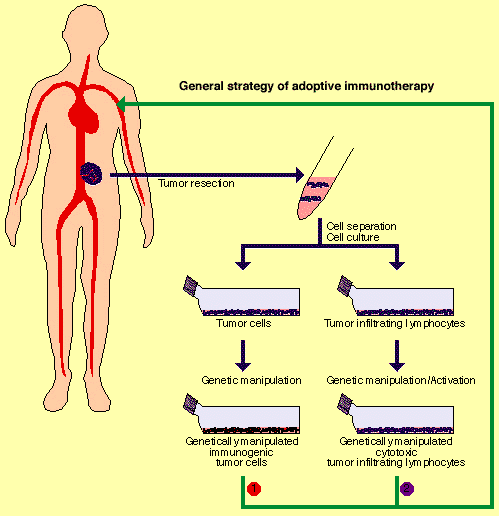
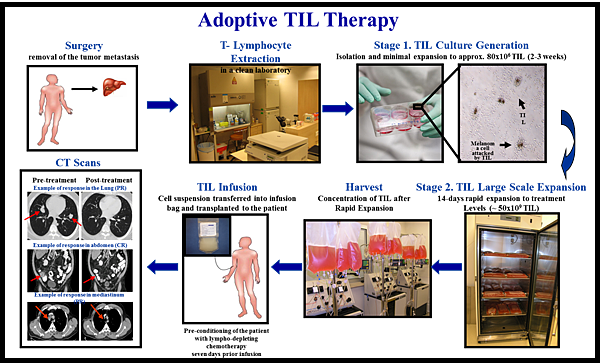
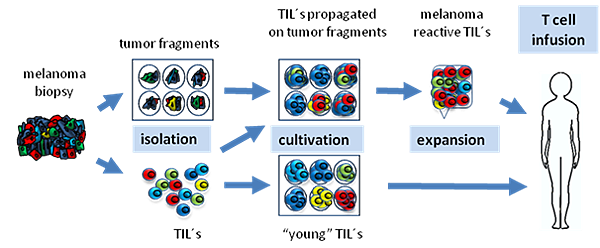
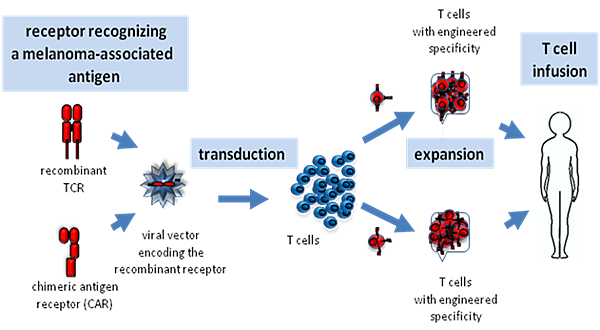
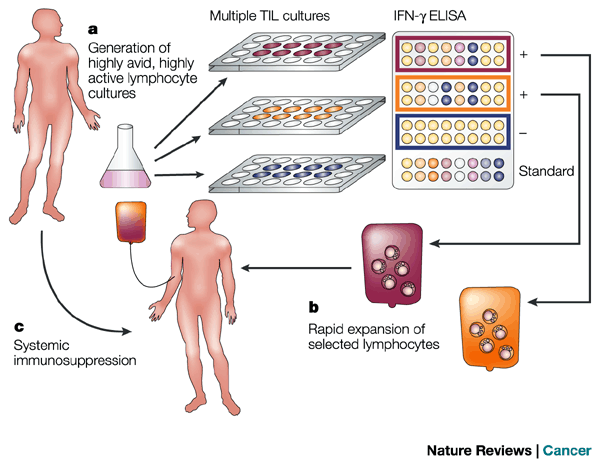
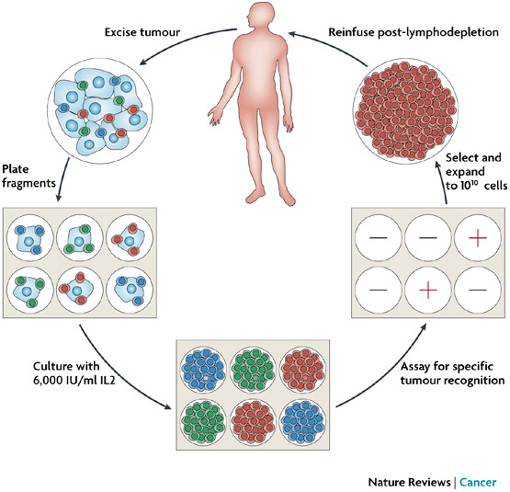
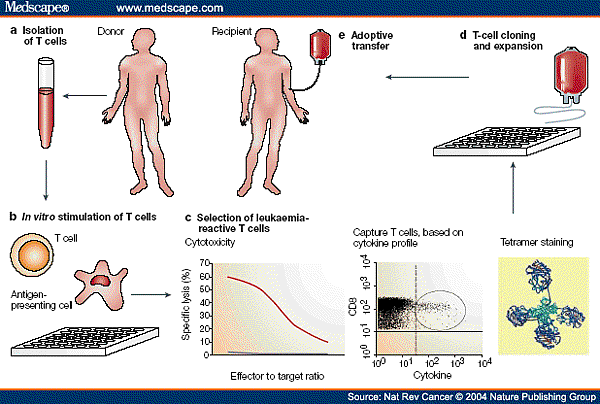

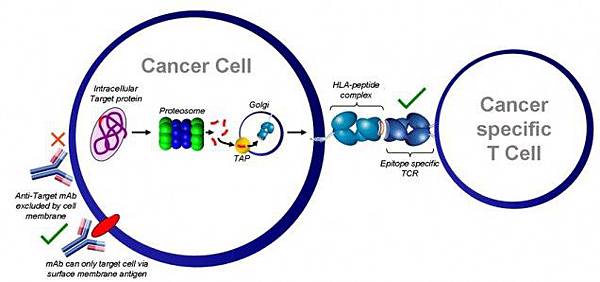
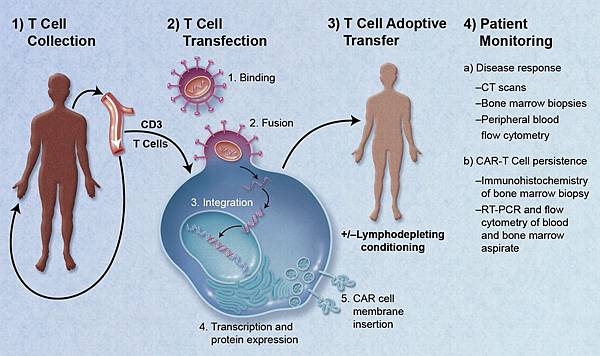
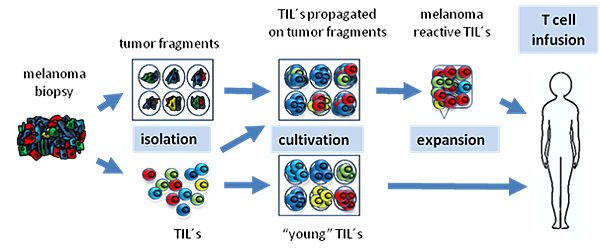
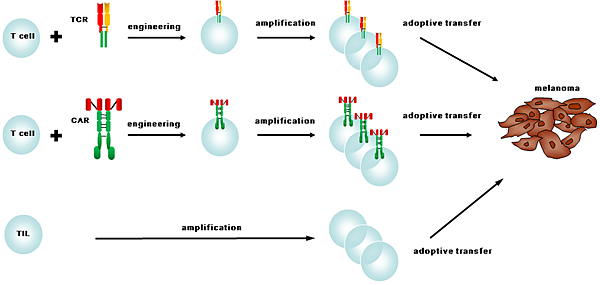
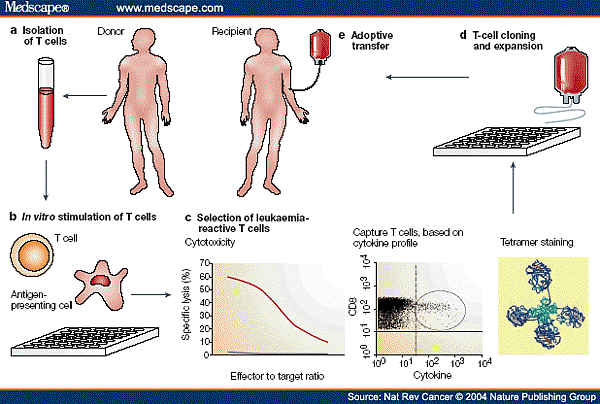
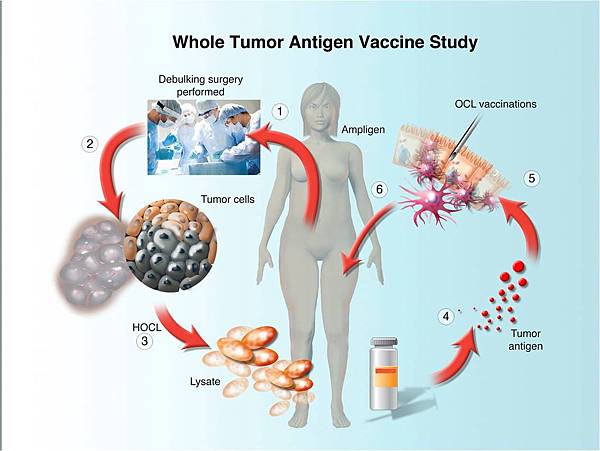
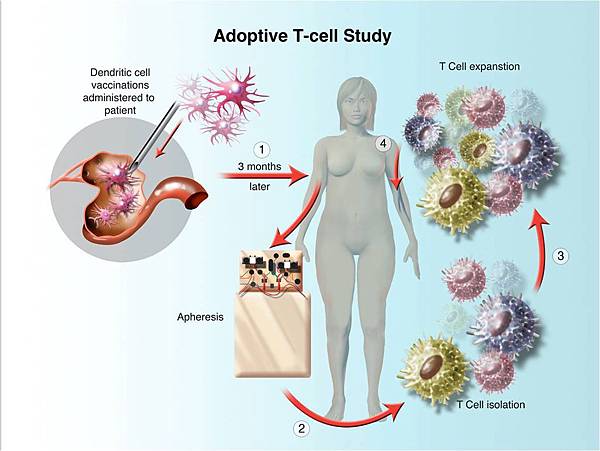
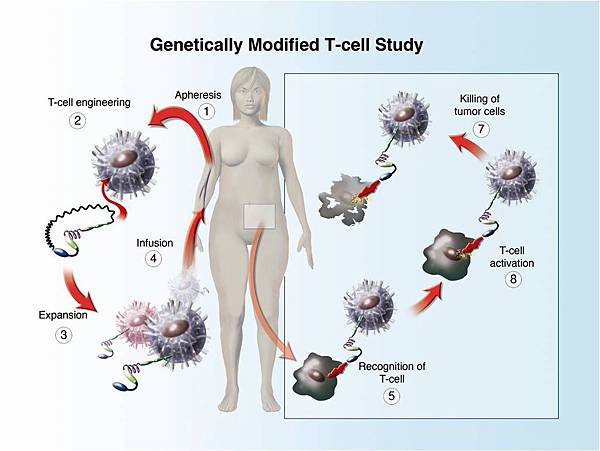
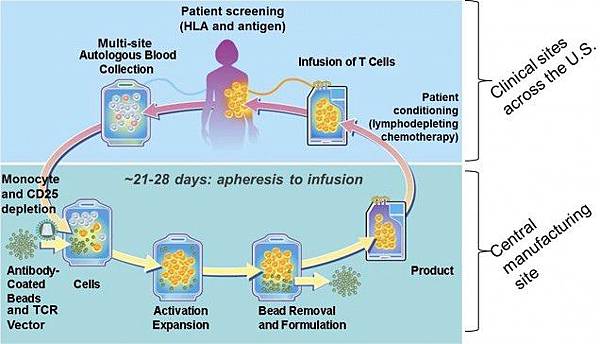
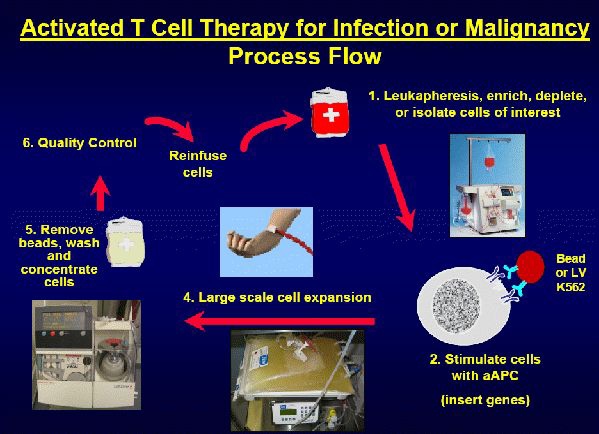

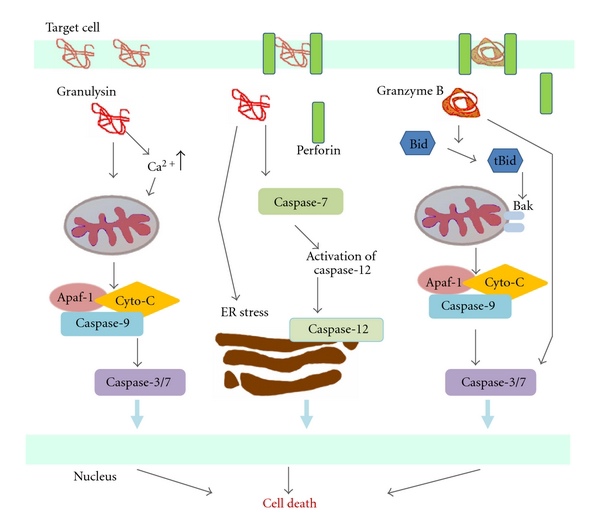
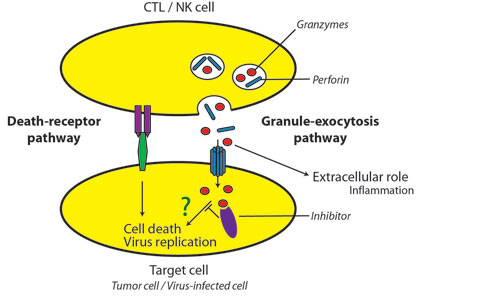
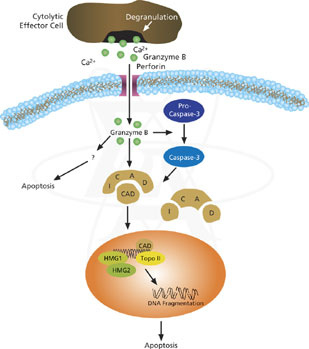
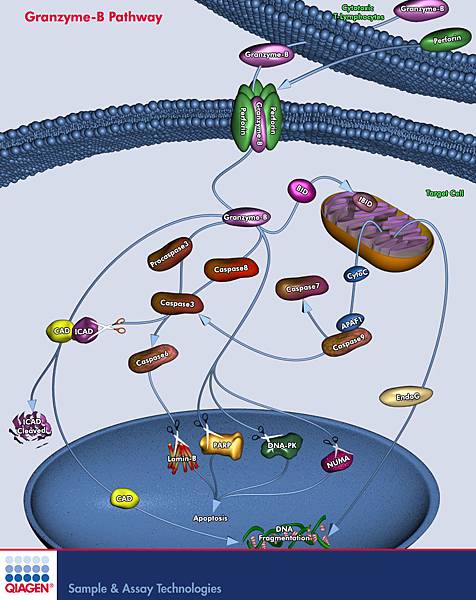
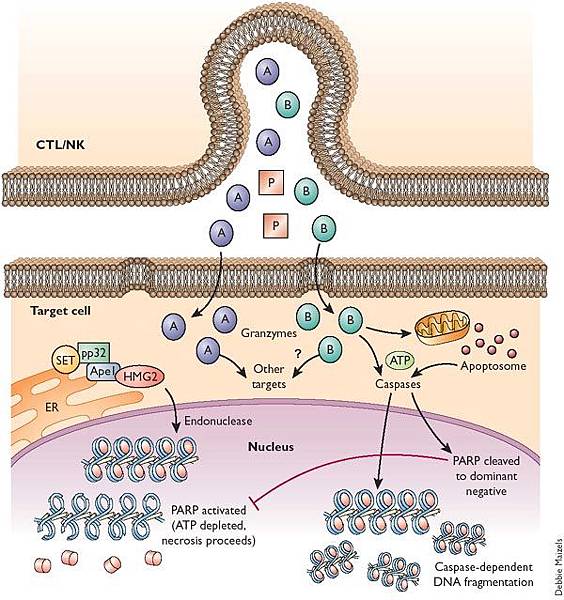
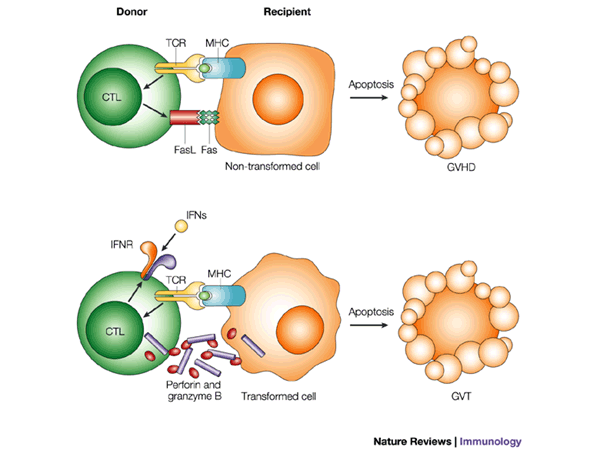
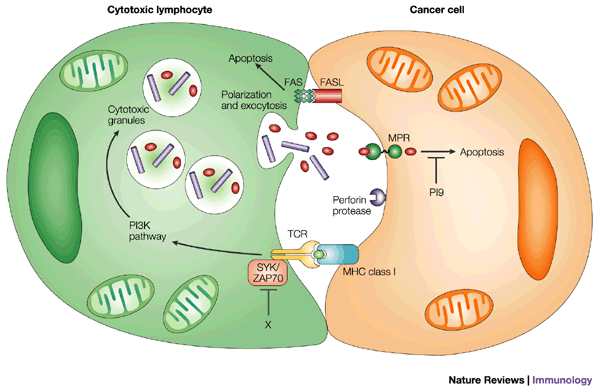

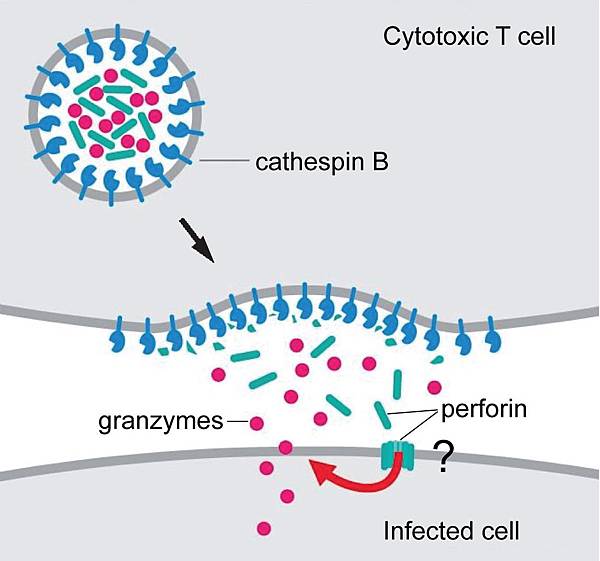
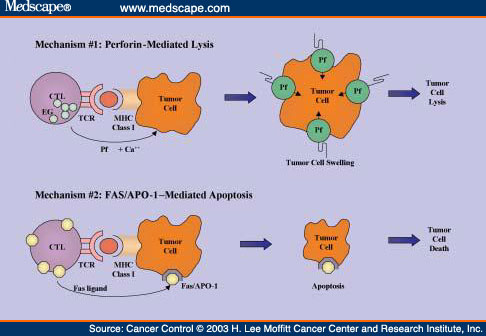
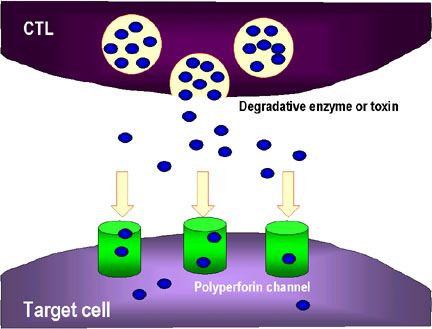
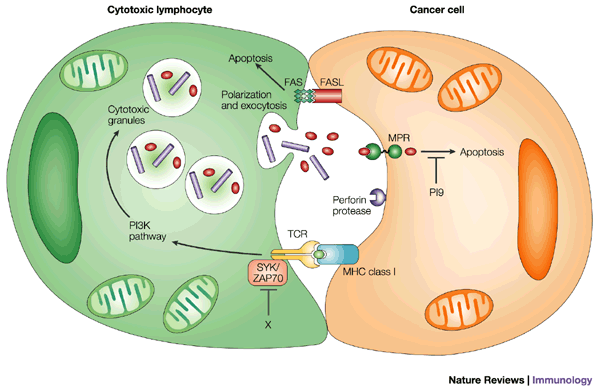
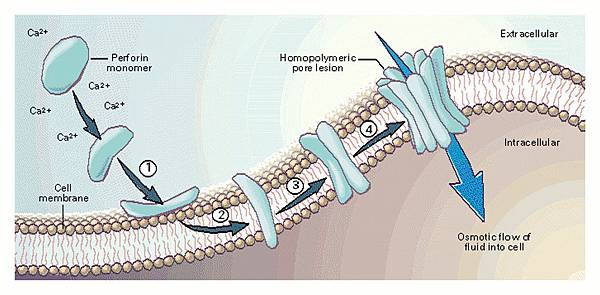
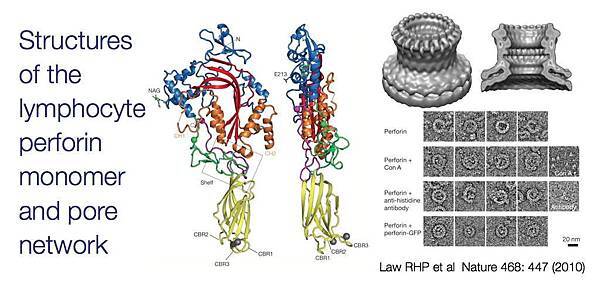
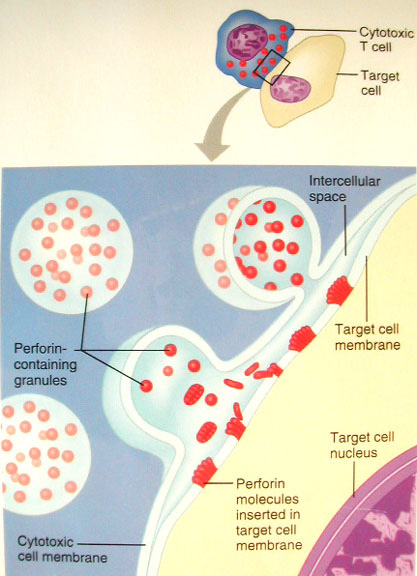








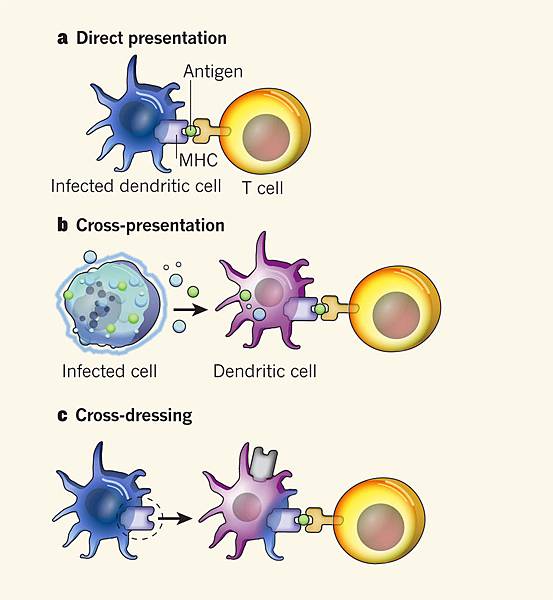

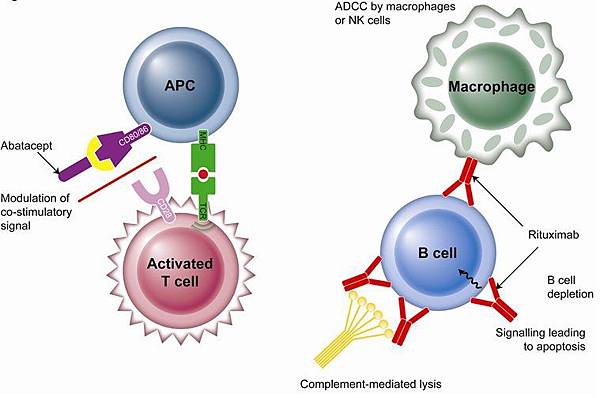







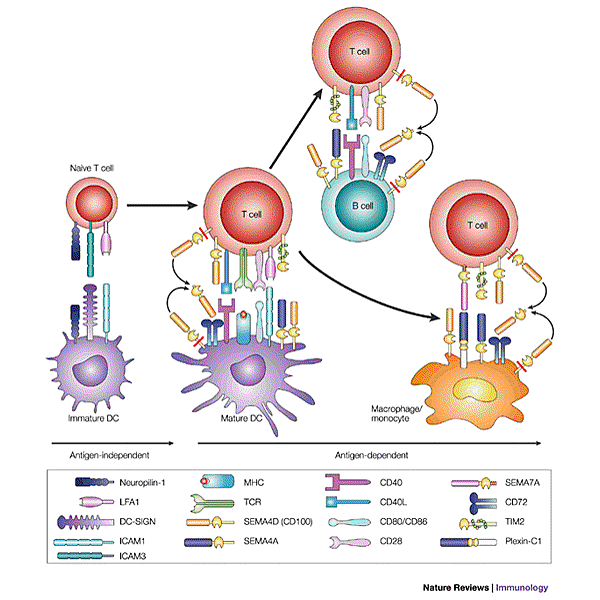











![tech_diagram[1] tech_diagram[1]](https://pic.pimg.tw/geantsage/1390205851-83606281.gif)







